ALLSTAR PROJECT
Holter monitoring tests typically record electrocardiogram (ECG) signals continuously for 24 hours; a 24-hour recording contains approximately 100,000 beats of ECG waveforms in an adult, and the amount of information that can be obtained from this is enormous. Currently, the Holter ECG is used in medicine to test for arrhythmia and transient myocardial ischemia (e.g., angina pectoris), but only a fraction of what it contains is being utilized. In recent years, with the development of computer science, techniques have been developed to analyze the information contained in the Holter ECG in detail, and it is now possible to obtain a variety of biological information that cannot be captured by conventional methods. These new indices can capture temporal changes in the organism in daily life, and thus include the activities of the nervous, immune, and endocrine systems, as well as changes in natural and social environmental factors that may be affecting these activities. From this perspective, the ALLSTAR project is collecting hundreds of thousands of Holter ECG data recorded throughout Japan and constructing a time-series database to analyze the effects of environmental factors on Holter ECG indices. This project is expected to further enhance the value of Holter ECG research in the medical and health sciences.
PROJECT OBJECTIVES
The purpose of this project is to establish a new evaluation method for the impact of environmental factors on health and disease from the database of Holter electrocardiograms recorded throughout Japan and to contribute to the promotion of predictive and preventive medicine and health promotion.
RESEARCH THEMES
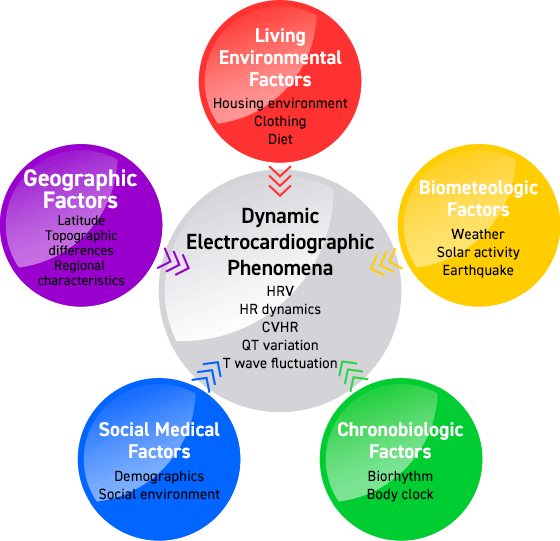
Examine the relationship between the following Holter ECG information, time/environmental information, and medical information
- ECG information: heart rate, heart rate variability, heart rate dynamics, arrhythmia, transient myocardial ischemia, ECG waveform variability
- Temporal and environmental information: weather, geomagnetic, lunar, solar activity, geographic information, and information about social conditions at the time of measurement
- Medical information: age, gender, clinical diagnosis, complications, medications, artificial pacemaker, symptoms, 12-lead ECG findings, cardiovascular risk factors, medication and behavioral records
PRIVACY POLICY
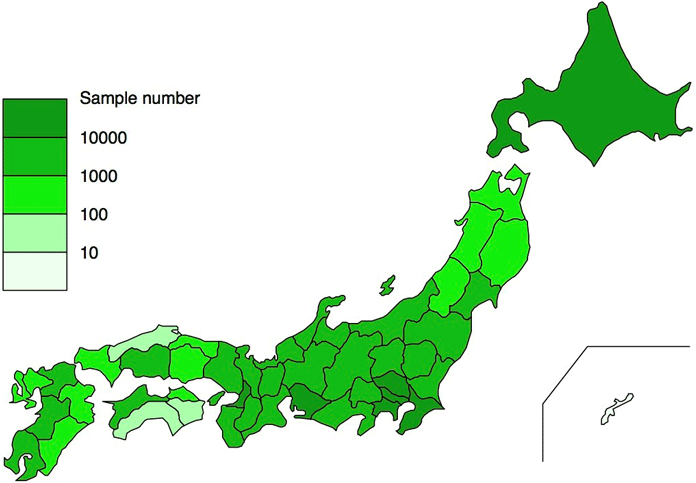
- Existing and future Holter ECGs analyzed since June 2008 at Suzuken's Holter ECG Analysis Centers (Tokyo, Nagoya, Sapporo) and their ancillary information will be used.
- These data will not be used in this study if the patient in question requests that they not be used in this study.
- The data will be anonymized using a unique research number so that the source of the data cannot be identified and will only be used by the ALLSTAR Study Group for the research described above. The correspondence between the name and the unique research number will be kept confidential by Suzuken Corporation and will not be disclosed to outside parties, including the members of ALLSTAR Study Group.
ORGANIZATION
ALLSTAR Study Group
- Junichiro Hayano
- Nagoya City University
- Norihiro Ueda
- Nagoya City University
- Itsuo Kodama
- Nagoya University
- Kaichiro Kamiya
- Nagoya University
- Takashi Kawamura
- Kyoto University
- Yoshiharu Yamamoto
- The University of Tokyo
- Eiichi Watanabe
- Fujita Health University / Bantane Hospital
- Kazuo Yana
- Hosei University
- Ken Kiyono
- Osaka University
[ Secretariat ]
- Emi Yuda
- Tohoku University